
Recent Advances in Protective Coating of Crude Oil Storage Tanks
| – Conference: The 6th Libyan Corrosion Conference, Tripoli, Libya, – November2007, Pp 460-472. – Authors: Mohamed Abdelgawad Gebril , Farag Shuaeib – Source: researchgate.net – Recent Advances in Protective Coating of Crude Oil Storage Tanks |
ABSTRACT
As coating developments are occurring every year. Therefore, a recent advances
investigation and analysis is necessary at certain milestone intervals. Furthermore, actual
experience feed back about the advantages and/or shortcoming of certain product or
design would be beneficial. The oil and gas particular field and more particularly the
storage tank coating systems are considered in this study. At the beginning, tank
corrosion problems are outlined and then a comprehensive theoretical characterization
and description for the major types of coatings is presented. The selection bases are also
stated for each type of coating. Then a crude oil floating roof storage tank case study has
been undertaken which illustrates the coating selection and discuses the current tank
coating problems. In this case study, coating selection was based on two approaches. The
first is by referring to the approved coating suppliers, and the second is by referring to
current coating specification used by two major oil and gas local companies. Selection
results from the two approaches are then compared and discussed. Research findings are
then concluded.
1. INTRODUCTION
Steel Storage tanks are used to store fluids such as crude oil, intermediate and refined
products, gas, chemicals, waste products, aqueous mixtures, and water. Corrosion is the
prime cause of the deterioration of steel storage tanks and accessories; therefore, control
and prevention of tank corrosion is of prime importance for efficient plant economics and
safety. One of the most efficient methods of tank corrosion prevention is by applying a
suitable coating. Therefore, the aim of this article is to provide up-to-date technological
directions in this field and compare them with what is currently being used by some of
the local companies. However, to begin with it was found worth to provide a brief
overview of tank corrosion problems which is usually encountered in the oil and gas
plants.
Tank corrosion can either be external or internal. Figure (1)-a, shows the areas of
corrosion for lower side of the tank, while Figure (1)-b, shows the areas of corrosion on
top side according to the tank roof type. External corrosion of tank bottoms can be
significant. The foundation material used for forming a pad under the bottom may contain
materials that are corrosive. For example, cinders may contain sulfur compounds that
become very corrosive when moistened. The presence of clay, wood, gravel, or crushed
stone as a contaminant in a sand pad may cause pitting corrosion at each point of contact.
Faulty pad preparation or poor drainage may allow water to remain in contact with the
tank bottom. If a tank previously leaked corrosive fluid through the bottom, accumulation
of the fluid under the tank can cause external corrosion of the tank bottom. For tanks that
are supported above grade, as shown in Figure 1a, an improperly sealed ringwall may
allow moisture to accumulate between the tank and the support, thereby accelerating
corrosion [1]. External corrosion also occurs when external insulation picks up ground
water, or when damaged or improperly sealed openings around nozzles and attachments
allow water ingress. Atmospheric corrosion can occur on all external parts of a tank. This
type of corrosion may range from negligible to severe, depending on the atmospheric
conditions of the locality. A sulfurous or acidic atmosphere can damage protective
coatings and increase the rate of corrosion. External surfaces of the tank and auxiliary
equipment will corrode more rapidly if they are not protected with paint or other
protective coatings or with cathodic protection where surfaces are in contact with
moisture. Continuous water contact due to pockets or depressions will be likely to cause
localized corrosion. Areas susceptible to this should be coated with coatings designed to
withstand immersion. The type of tank and the construction details used can affect the
location and extent of external corrosion.
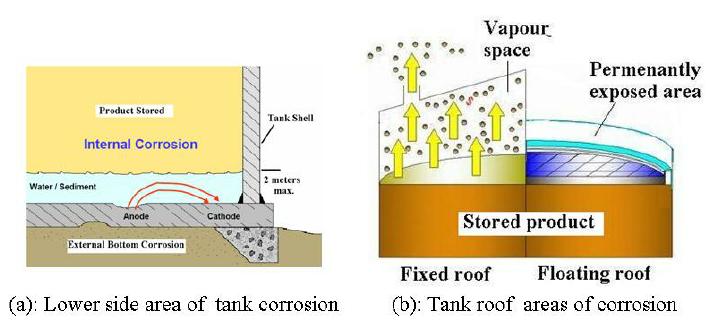
The occurrence of internal corrosion of a storage tank depends on the contents of the tank
and the material of which the tank is constructed. In some cases it is necessary to use
linings (coatings) that are more resistant to the corrosive properties of the stored fluid
than are the tank construction materials. In some particularly corrosive services, it may be
necessary to construct the tanks of a corrosion resistant material [1].
Crude oil and petroleum product tanks are usually constructed of carbon steel. Internal
corrosion in the vapor space above the liquid of the fixed roof tanks is commonly caused
by hydrogen sulfide vapor, water vapor, oxygen, or any combination of the three (See
Figure 1-b). In the areas covered by the stored liquid, corrosion is commonly caused by
acid salts, hydrogen sulfide or other sulfur compounds, or contaminated water that settles
out with solids on the bottom of the tank. This bottom layer is typically referred to as
bottom sediment and water (BS&W) as shown in Figure (1)-a. Coating of tank surface,
particularly paints and lacquers, is by far the most important of all methods for corrosion
prevention and probably accounts for about half of all costs spent on anti-corrosion
measures.
2. PROPERTIES OF PAINTS
Generally, the most obvious properties that a paint must have are shown by the sketch of
Figure (2):
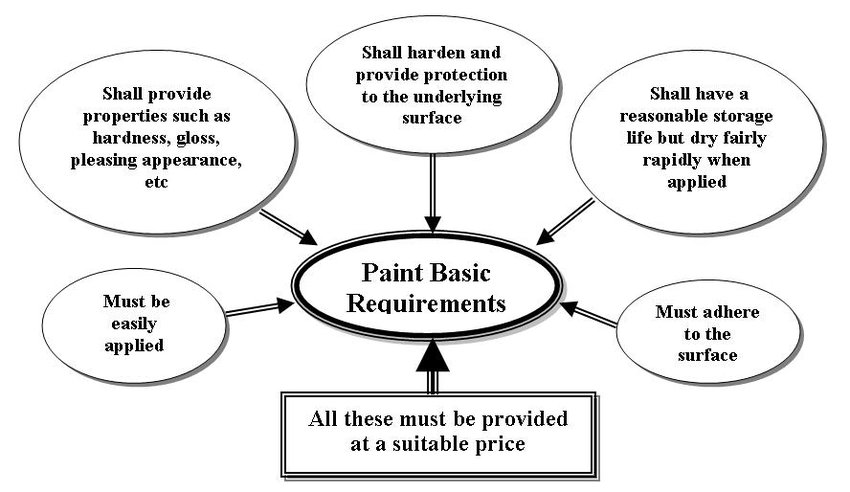
Often, a single paint will not have all the required properties so a number of coats, or
paint system, will be applied rather than a single coat. The first coat or primer must
protect and adhere to the tank surface (The paint teeth). The paint film is built up by
intermediate coats and a top coat or finishing coat provides protection against sunlight,
abrasion, etc and often provides decoration. Primers and topcoats must, of course, be
compatible with one another, and coating suppliers should be consulted for such
information. Due to application costs being far higher than material costs, the move is to
reduce to a minimum the number of coats in a paint system with a single high-build
system.
Paints basically consist of solid particles, called pigments, dispersed in a liquid, known
as the binder, medium, film-former or vehicle. When the paint dries, the binder binds the
pigment particles together in a coherent film that adheres to the surface. The binder
provides most of the properties required to protect the substrate from the environment,
and is the most important constituent of the paint. The binder generally consists of an
organic polymer known as a resin dissolved in a solvent. Paints are generally
categorized according to the binder type, with names such as alkyd, epoxy,
polyurethane, etc. The pigment provides corrosion protection properties along with color
to the film (binder). In addition to binder and pigment, most paints contain additional
solvent (thinner) to thin the paint out so it can be more easily applied. Solvents
completely evaporate and play no part in the dry film. Other additives include extenders,
which are similar to pigments and added as a pigment to build up solid constituent or to
modify paint properties. Other additives include driers, anti-skinning agents and
thixotropic agents which reduce sagging of the paint when applied to a vertical surface.
Paints protect basically by providing a barrier between the substrate and the
environment. They are electrically insulating which impedes the movement of the ions
which take part in the corrosion reactions. Water and oxygen can both diffuse into paint
coatings, although some are more impermeable than others and therefore provide greater
protection.
An inert or barrier pigment such as iron oxide assists in this barrier effect. Leafing
pigments, such as micaceous iron oxide (MIO) and leafing aluminum, provide an
overlapping, shingle effect, providing a further barrier to the corrosive elements and
strengthening the coating. Such pigments also protect the binder from UV
degradation. Aluminum is also used for high temperature paints, but should not be
used where chemical resistance is required. Such pigments are best used in top coats
but can be added to primers. Lamellar pigments will affect the color and appearance
of the film. In addition to the barrier effect, primers usually contain pigments which
can provide additional protection. Inhibitive primers contain chemical compounds
which dissolve in water to some degree and form a protective passive chemical
compound at the anodic sites on the metal. This reduces or prevents corrosion, but
such pigments are only used in primers for atmospheric conditions, not for immersed
environments where they can give rise to osmotic blistering. Red lead and zinc
chromate both provide an inhibitive effect, although primers containing such
compounds are no longer used due to their toxicity. These pigments have now been
replaced by zinc phosphate, although this pigment probably provides protection more
by barrier than inhibitive effect. Zinc-rich primers provide a barrier effect but, more
importantly, have sufficient metallic pigment (90 per cent or more in the dry film) for
them to be able to provide cathodic protection. The zinc powder is in sufficient
concentration for there to be a conducting path between the zinc particles and the
steel. Clearly, the surface must be well prepared so there can be electrical contact
between the zinc and the steel. At a scratch in the coating, the zinc corrodes in
preference to the steel. Additionally, zinc corrodes at a significantly lower rate than
steel and the zinc corrosion products which form tend to block up the pores in the
coating providing further barrier protection [3].
3. MAJOR TYPES OF PROTECTIVE COATINGS
There are several types of paint which are commonly used in oil and gas plants.
Applicable paints such as: (1) Acrylic, (2) Epoxy, (3) Vinyls, (4) Alkyds, (5)
Chlorinated rubber, (6) Coal Tar Epoxies, (7) Polyurethane, (8) Silicone resins, (9)
Polyester and vinyl ester, (10) Zinc-rich paints. Only types with more interest to crude
oil storage tank painting will be described in details hereafter. Other paints details cab be
obtained from reference [2-3].
Acrylic: coatings are derived from acrylic and methacrylic acid and, as there are
many possible polymerization reactions which can occur, there are almost unlimited
ranges of materials available varying from hard, brittle materials to soft, flexible
coatings. They are characterized by excellent color retention; ultraviolet stability and
gloss along with resistance to chemicals, moisture and weathering so are widely used
as top coats. They are available as paints which dry by solvent evaporation which are
mainly used for production line applications, such as automobiles, and as water-based
emulsions which are becoming increasingly important for top coats of structures not
located in aggressive environments. Two-pack or catalyzed acrylics are also available
which are harder, tougher and more heat and solvent resistant than the other types and
can be used as top coats in more aggressive environments. Because of their cost and
health and safety advantages, these are commonly specified where polyurethanes may
have been used in the past. They are generally not used for immersion service or in
strong chemical environments.
Epoxy: systems are generally two-pack convertible products which cure by chemical
reaction forming a film which is strongly adherent, hard and chemical and solvent
resistant. Epoxies are commonly specified where long term protection and
performance is required and where high standards of surface preparation can be
achieved. While alkyds are the workhorse coating under mild to moderate conditions,
epoxies are the workhorse under more severe conditions. In common with other twopack coatings, they require proper mixing before application, there is a limited time
during which the paint can be applied and curing time is strongly temperature
dependent. Many epoxies will not cure below 5 deg C, although epoxies which cure
below this temperature are now available. There are two main types of curing agents
used with epoxies. Polyamide-cured epoxies have better weathering, are more
flexible, cheaper, mixing ratios, are less critical and have the longer pot life, although
this means a longer curing time. Polyamide epoxies are more forgiving of
condensation or high relative humidity during curing. Polyamine-cured epoxies have
better chemical and solvent resistance and are generally used for specialist purposes
such as tank linings. Most are now amine-adduct curing types which means the amine
is partially reacted with the epoxy resin giving a material which is easier to mix and
apply than the straight amine cured epoxies. Epoxies are prone to loss of gloss on
exposure (chalking) although their other properties are unaffected. Often a
polyurethane or acrylic finish coat is specified for epoxies to avoid this problem.
Epoxies can be used as primers, where they can be pigmented with zinc dust, zinc
phosphate or MIO. As intermediate or finishing coats they can be pigmented with a
full range of colored pigments or MIO. They are widely used for offshore structures
and for other aggressive and chemically polluted areas. Maintenance with these
coatings is often difficult since they are hard and tend to harden further on ageing. A
light blast clean is generally required to roughen an old epoxy coating to allow the
new coating to adhere. There have been a number of developments in coatings based
on epoxy resins. Solventless, Ultra High Build (UHB) or high solids epoxies are
available which allow high film builds of a millimeter or more in thickness to be
applied. High build epoxies, as distinct from high solids epoxies, contain inert
pigments which provide an inert, impervious coating which is often used for tank
linings. Epoxy mastics have been developed which are tolerant to hand prepared rusty
steel surfaces. Specially formulated polyamide-cured epoxies have the ability to
displace water from the substrate and such materials can even be applied and cured
underwater. Epoxies can be modified with other resins such as silicones, phenolics or
urethanes to produce improved water, chemical and solvent resistance. Water-based
epoxies are now available which provide the environmental and health and safety
advantages of water-based coatings, although generally do not provide as good
corrosion resistance as solvent-borne epoxies. Epoxy esters are not true epoxies, but
rather single-pack alkyds modified with epoxy.
Coal Tar Epoxies: are epoxies which have been modified with coal tar providing
the advantages of epoxies with improved water resistance. They are less resistant to
solvents than conventional epoxies and only available in dark colors. Because they
degrade from UV radiation, they are not normally used in atmospheric exposures.
They are widely used in sewage industry as they are resistant to hydrogen sulphide
and bacteria attack. They are compatible with cathodic protection and extensively
used for tanks, ships’ hulls and on offshore structures. However, due to the
carcinogenic nature and health issues of the coal tar, they are being phased out and
will be replaced by other coatings, generally conventional epoxies [4-6].
Polyurethane: coatings are based on the reaction between isocyanates and hydroxylcontaining compounds, usually an acrylic, epoxy, alkyd, polyester or similar
material. For protective coatings they are usually two-pack types and the isocyanates
are classified as either aromatic or aliphatic. Both types form a hard, tough film
which shows excellent chemical and solvent resistance, color and gloss retention and
will cure at lower temperatures than epoxies. However, they are more expensive than
epoxies, and more difficult to handle and apply as the resin is moisture-sensitive in the
liquid state and is toxic. Aromatic urethanes, such as toluene di-isocyanate (TDI) are
cheaper and suitable for immersion service. Aliphatic urethanes, hexamethylene diisocyanate (HMDI) for example, have better color and gloss retention. Unlike most
coatings which chalk and yellow after prolonged exposure, aliphatic urethanes
continue to maintain a glossy, `wet look’ years after application.
Because of their hardness, maintenance is difficult as a new coat will not adhere.
However, recoatable polyurethanes modified with acrylic resins have been developed
which are easier to recoat. Moisture-curing urethanes are one-pack materials that
react with atmospheric moisture to cure. These are rapid cure coatings which can be
applied at low temperatures and under adverse weather conditions. However,
moisture must be completely excluded from the raw material and its rapid cure
means overcoating must be carried out quickly or problems with intercoat adhesion
will arise. Elastomeric urethanes are flexible, rubbery coatings which have excellent
adhesion and abrasion resistance, along with resistance to chemicals and weathering.
They are expensive but ideal in abrasive and corrosive conditions. So-called
`isocyanates-free polyurethanes’ are not polyurethanes but catalyzed acrylics.
Polyester and vinyl ester: resins are best known in the reinforced plastics industry
where they are used as binders for glass fibers or other materials to form a range of
products. In the protective coatings area, they are used to form a thick, hard, acid
resistant coating which is often used for tank linings. They are fast curing, but this
makes them difficult to apply. They shrink on curing so are often reinforced with glass
fibers or flakes. They are brittle and should not be used under alkaline conditions since
both the resin and glass break down in such environments. Vinyl esters tend to give
better alkali resistance and adhesion than the polyesters. Both types are often used as
linings for tanks and chemical process equipment.
Zinc-rich paints: have a variety of binder types, but because of their importance in
corrosion protection, are worth considering as a generic group. They all have a high
proportion of metallic zinc powder in the dry film which provides protection by cathodic
protection and barrier action as described above. In addition, zinc corrosion products
(carbonates, hydroxides, etc) fill and seal pores in the coating. Zinc rich paints can have
organic or inorganic binders, although there are a number of categories within each
group. Zinc rich coatings should not be used in aggressive acid or alkali environments
(pH outside the range 6 to 10.5).
Organic zinc-rich: coatings can be divided into single pack and two pack types and can
use virtually any binder that can resist the alkaline zinc corrosion products. The single
pack types have binders made from resins such as chlorinated rubbers, acrylics and
vinyls and are easier to use, but have limited application in the protective coatings field
as the protection provided by them is limited. Use is usually restricted to repairing
damaged and welded areas on galvanized steelwork. Two-pack types are usually based
on epoxy resins, although polyurethanes can be used. These are more resistant and
widely used as primers under atmospheric conditions. Organic zinc-rich paints are
generally easier to use than the inorganic variety, and are somewhat more tolerant of
poor surface preparation. However, they do not provide the protection and durability
afforded by the inorganic type.
Inorganic zinc rich: coatings comprise zinc metal in a silicate binder. As an inorganic
material, such binders have vastly superior weather, abrasion, heat and solvent resistance
over even the best organic binder. In addition, the cement-like nature of the coating
means they have a much greater coefficient of friction than other paints and can be used
on surfaces connected by high-friction grip bolts. Such coatings are generally quick
drying and also provide excellent resistance to humid and marine atmospheres. There are
some problems associated with their use. They are not resistant to acids and alkalis, and
require excellent surface preparation. They must be sprayed and must not be applied too
thickly or mud cracking will occur. They will provide excellent protection on their own,
but can be top coated for additional protection or aesthetic reasons. When overcoating
inorganic zincs, the top coat must be compatible with the zinc and the surface must be
thoroughly cured and clean, or adhesion problems may result. Because of its porous
nature, it is usually advisable to apply a thin, sealer coat before application of the top
coat.
4. CRUDE OIL TANK COATING SELECTION (CASE STUDY)
Based on the previous comprehensive theoretical background on the coating materials,
their applications and selection, a case study is considered in this work inorder to provide
complete details for a tank coating issues. The protective coating system required is for a
20m diameter by 10m height floating roof steel tank for crude oil storage. The location
considered is in an oil terminal at the coast side of Libya. The crude oil is API 35 and the
maximum operating temperature is expected to be 60 Co (140 o F) and the design water level in the tank is 1 meter height. The tank is sand blasted to SA 2 ½ (ISO 8501-1:1988).
For crude oil floating roof storage tanks, surfaces to be coated is categorized as internal
and external. Internal include the tank internal bottom and 1 to 2 m height from the tank
shell. And external surface include the external tank shell and the 1 to 1.5 m area of the
permanently exposed internal surface of the tank shell measured from the top of the tank.
Tank external bottom is not painted due to cathodic protection requirements. This
problem is talked by two approaches; the first is by referring to the recommendation of
four coating suppliers who are approved by local oil and gas companies, and the second
is by referring to the current local companies coating specification and adopted
procedures. An interviews and/or official contacts were made whenever necessary
particularly on the local companies’ side. Coating suppliers were referred to from their
available in hand catalogues (some might need updating), or from their internet web sites.
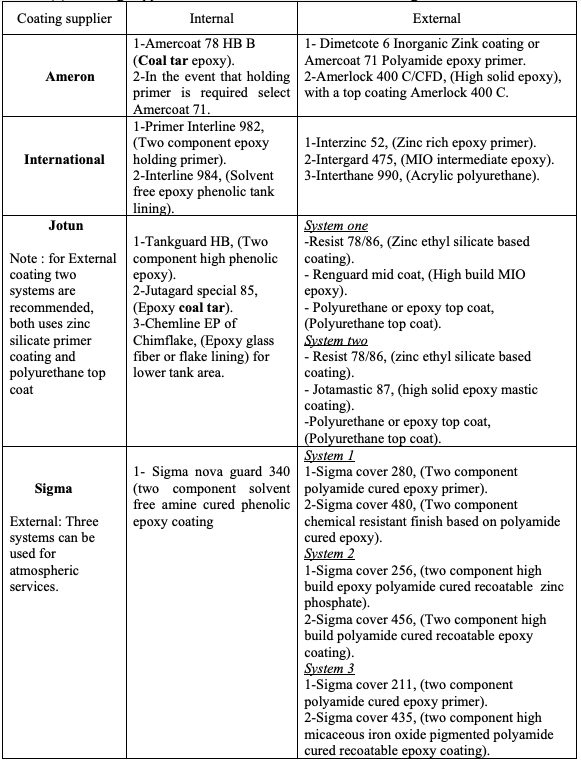
4.1. Coating Supplier Recommendation for Tank Coating
The considered companies deal directly with the oil and gas industry and have developed
coatings especially for this field. We will be presenting the products that they distribute
to North Africa generally and to Libya especially. Four well known and approved coating
suppliers are considered in this study, they are [7-10]:
1) Ameron 2) International 3) Jotun 4) Sigma
There are other high quality coating suppliers, but in general these are the most popular in
Libya. Table (1) shows these suppliers recommendation. As shown, for the external
coating, except Sigma, the most common coating types are:
1- Primer: Zink silicate epoxy
2- Intermediate coat: Epoxy (MIO or high solid)
3- Top coat: Polyurethane
However, according to Table (1) for the internal coating, there is a significant conflict
among the four suppliers. Both Ameron and Jotun still recommend the coal tar epoxy
while International and Sigma do not recommend this type of coating. International
supplier has been contacted and confirms that they do not producing this coating anymore
due to health issue [8]. The available data from Ameron and Jotun might be out of date
and a confirmation needs to be made to investigate their position from this issue. Another
point of difference is that Ameron and Jotun specifically recommends the fiberglass mat
or flakes composite layer for the tank bottom and 1 meter height of the tank shell.
Table (1): Coating suppliers’ recommendation for crude oil storage tank.
4.2. Coating Selection Based on Coating Specifications of Local Companies
There are several Libyan companies that extract, store and refine oil, the following
companies are from the biggest and the most important:
1- The Arabian Gulf Oil Company (AGOCO).
2- SIRT Oil Company.
The general coating specification form these two companies were reviewed and their
recommendations for crude oil storage tank are outlined in Table (2) [11-12].
Table (2): Local companies coating systems according to their coating
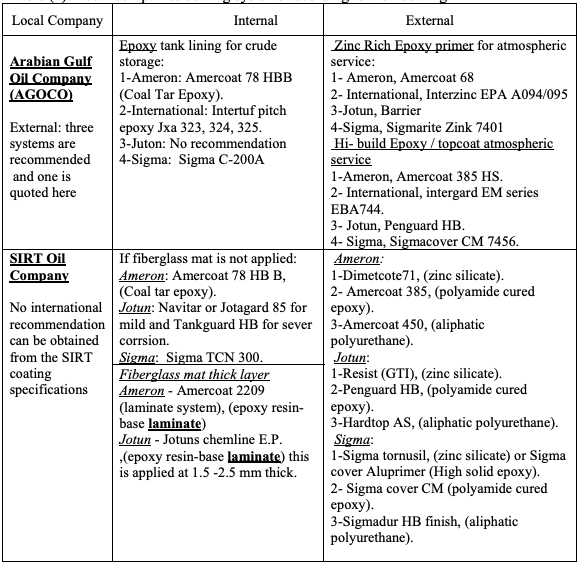
Regarding local companies, as can be seen, for the internal coating both AGOCO and
SIRT still recommend epoxy coal tar coating which need to be reviewed. Also, AGOCO
dose not uses the laminated fiberglass internal bottom lining anymore. This issue have
been discussed with the company engineers and explained that their suppressing of the
composite laminate usage was due to both cost and maintenance reasons. They confirmed
that the internal fiberglass composite laminate, if made properly, would prevent corrosion
from the internal side for a longer period, but in case of tank leak it will make inspection
of the bottom plates very difficult if not impossible. This will also make patch repair not
feasible unless the fiber mat is removed which is a difficult and costly task. In such
situations usually a decision to remove the whole bottom would be made. The other point
is that according to company experience most of the problems of the tank bottom came
from the external side of the tank due to soil problems. However, SIRT Oil Company still
using this type of lining and they have confirmed that they have no problems with using
this type of coating. The conflict between the two local companies’ choices might be
attributed to the soil condition being at SIRT performing better than that at AGOCO
locations. In this case we can conclude that unless the soil condition is corrosive the fiber
glass mat may be better except for inspection requirements. This issue seems will be
under debate for a while and need further analysis.
For the external side paint there are not much differences and the choice is similar for
both companies. However, it is to be remarked that Sigma recommendation by SIRT Oil
Company are different from all the three systems recently recommended by the supplier
(See Table 1) which might be attributed to Sigma supplier product developments, and
more specifically their use of two component paints which is reported to have a cost
advantages.
Also, as shown in Table 2, the local companies specify, in their coating specification, the
trade name of the coatings. By comparing these with the coating suppliers names, some
of them were found different, and upon contacting one of these suppliers, they replied
that some coating are either renamed or not produced any more and this is a routine
procedure quite often every now and then. However, local companies’ coatings and
construction specification, once approved, are normally used by the engineering or
projects department. These specifications are anticipated to be subjected to a routine
review at specified interval, usually not less than three years. However, corrosion section
who usually approve the coating specification is generally under the maintenance
department (such as at AGOCO). Therefore, any significant development is expected to
be known by the corrosion section and reported to the engineering department, but it
might not be clearly added to the company general specifications. This might led to what
is happening currently of still existence of the « Coal Tar Epoxy » coating, which is
reported to have some health hazards.
4.3. Tank Bottom External Side
A standard practice in most of the Libyan oil and gas companies is not to coat this side
due to cathodic protection requirements, which is agreed. However, less concentration
seems to be given to the backfill material underneath the tank bottom. Some construction
specification calls for asphalt pad surfacing material to be put at the top of the backfill
material. The surfacing material is 80 mm layer of hot-mix-cold laid bituminous asphalt
cover over a prime coat. This layer should not be done if cathodic protection would be
installed as it would work as insulation for the CP protection current (see Figure 3).
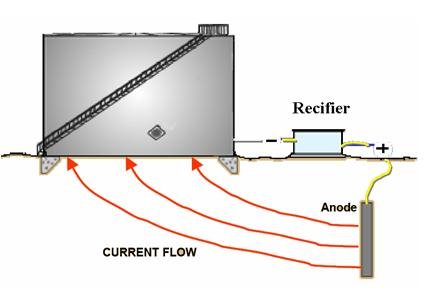
Furthermore, most specifications ask for compacted salt free sand which is again will do
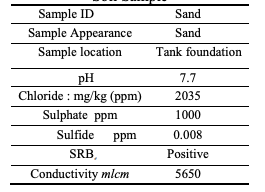
very well. However, two issues can be emphasized here, the first is that some locations as the one under consideration, may have bacteria (see Table 3), which mean that the tank area soil need to be replaced totally. The second issue is that both salt and bacteria testing
of the backfill material need to be clearly stated in the tank construction specifications.
As if not clearly stated contractors will not be obliged to do them.
5. CONCLUSIONS
Therefore, from this work, the general coating characteristics are described and the
particular tank coating selection requirements is highlighted. A case study to apply the
previous coating selection theory has been undertaken and two coating selection
approaches were followed; the first is by referring to four well known coating suppliers
and the second by referring to two local oil and gas companies specifications. This
allowed most of the related information gathered for further analysis and discussions.
Some of these efforts finding are:
1- Regarding the internal side, coal tar epoxy application is suppressed internationally
and need to be reviewed by local companies due to health hazards.
2- The tank water and basic sediment area is covered by either paint coating supported by
CP system or thick fiberglass laminate. A conflict of requirements currently exists
between coating suppliers together and also between local companies. This issue needs
further research and investigation even though the authors are convinced by the use of the
coating with CP option.
3- Regarding the external side, the Zink silicate epoxy primer, and the polyurethane top
coat showed more dominant usage. The intermediate coat is mostly epoxy either as a
single or two component coating.
4- According to AGOCO maintenance records it seems that most of the tank problems
are from the foundation side and, more focus should be given to it. Various conflicting
requirements for the backfill material do exist and need to be investigated and clarified.
Also, some of the specification of the tank soil call for mixing the backfill sand with
bitumen layer which proven to have diverse effect on the tank cathodic protection. The
type of soil requirements is said to be salt free soil. However, full chemical analysis
seems necessary as sulfide reducing bacteria (SRB) and other corrosive materials are
some times available and in such circumstances a totally new and clean backfill material
need to be used.
| – Conference: The 6th Libyan Corrosion Conference, Tripoli, Libya, – November2007, Pp 460-472. – Authors: Mohamed Abdelgawad Gebril , Farag Shuaeib – Source: researchgate.net – Recent Advances in Protective Coating of Crude Oil Storage Tanks |













